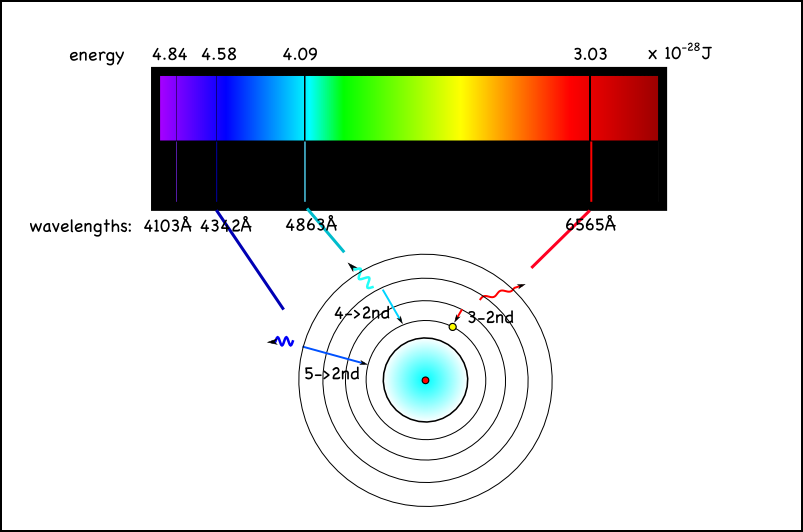
Light Emission Vs Absorption . the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. Absorption and emission are two common phenomena associated with electron transitions within. infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. the main difference between emission and absorption spectra is that an emission spectrum has different coloured.
from montessorimuddle.org
the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Absorption and emission are two common phenomena associated with electron transitions within. the main difference between emission and absorption spectra is that an emission spectrum has different coloured.
Emission Spectra How Atoms Emit and Absorb Light Montessori Muddle
Light Emission Vs Absorption Absorption and emission are two common phenomena associated with electron transitions within. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Absorption and emission are two common phenomena associated with electron transitions within. infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. the main difference between emission and absorption spectra is that an emission spectrum has different coloured.
From www.slideserve.com
PPT Lecture 12 Introduction to Atomic Spectroscopy PowerPoint Light Emission Vs Absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. when there is spontaneous emission (like in your case),. Light Emission Vs Absorption.
From www.researchgate.net
The interaction of light with matter via (a) absorption, (b Light Emission Vs Absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature. Light Emission Vs Absorption.
From www.britannica.com
Light Emission, Absorption, Processes Britannica Light Emission Vs Absorption the main difference between emission and absorption spectra is that an emission spectrum has different coloured. Absorption and emission are two common phenomena associated with electron transitions within. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. during absorption,. Light Emission Vs Absorption.
From sixdayscience.com
Chapter 7 SixDay Science Light Emission Vs Absorption Absorption and emission are two common phenomena associated with electron transitions within. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. the main difference between emission and absorption spectra is that an. Light Emission Vs Absorption.
From byjus.com
Emission spectrum Atomic spectra Chemistry Light Emission Vs Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. the main difference between emission and absorption spectra is that an emission spectrum has different coloured. Absorption and emission are. Light Emission Vs Absorption.
From chem.libretexts.org
14.1 Vocabulary Chemistry LibreTexts Light Emission Vs Absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. the main difference between emission and absorption spectra is that an emission spectrum has different coloured. The result is an emission. Light Emission Vs Absorption.
From www.youtube.com
Absorption spectrum and Emission spectrum video YouTube Light Emission Vs Absorption Absorption and emission are two common phenomena associated with electron transitions within. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. . Light Emission Vs Absorption.
From www.slideserve.com
PPT Light Emission Spectra & The Quantum Mechanical Model PowerPoint Light Emission Vs Absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Absorption and. Light Emission Vs Absorption.
From www.pinterest.com
Absorption and emission lines Astrophysics, Space and astronomy Light Emission Vs Absorption Absorption and emission are two common phenomena associated with electron transitions within. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. . Light Emission Vs Absorption.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Light Emission Vs Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. the main difference between emission and absorption spectra is that an emission spectrum has different coloured. Absorption and emission are two common phenomena associated with electron transitions within. infographic showing the relationship between the continuous spectrum of a star whose light. Light Emission Vs Absorption.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption Light Emission Vs Absorption infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. Absorption and emission are two common phenomena associated with electron transitions within. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of. Light Emission Vs Absorption.
From www.youtube.com
Difference Between Absorption and Emission Spectra YouTube Light Emission Vs Absorption the main difference between emission and absorption spectra is that an emission spectrum has different coloured. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. Absorption and emission are two common phenomena associated with electron transitions within. during absorption, an electron takes energy from an incoming photon, and the internal energy. Light Emission Vs Absorption.
From www.slideshare.net
Chapter 3 Arrangement of Electrons in the Atom Light Emission Vs Absorption the main difference between emission and absorption spectra is that an emission spectrum has different coloured. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. Absorption and emission are two common phenomena associated. Light Emission Vs Absorption.
From www.pinterest.com
Emission Spectrum Vs. Absorption Spectrum Astronomy lessons Light Emission Vs Absorption during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms. Light Emission Vs Absorption.
From www.youtube.com
A Level Physics Absorption and emission spectra explained YouTube Light Emission Vs Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. Absorption and emission are two common phenomena associated with electron transitions within. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. the main difference between emission and absorption spectra is that an. Light Emission Vs Absorption.
From www.youtube.com
Absorption vs Emission YouTube Light Emission Vs Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. when there is spontaneous emission (like in your case), the electron relaxes, emitting a photon. Absorption and emission are two common phenomena associated. Light Emission Vs Absorption.
From www.slideserve.com
PPT Chemistry XL14A Nature of Light and the Atom PowerPoint Light Emission Vs Absorption Absorption and emission are two common phenomena associated with electron transitions within. the main difference between emission and absorption spectra is that an emission spectrum has different coloured. the manner in which visible light interacts with an object is dependent upon the frequency of the light and the nature of the atoms of the object. infographic showing. Light Emission Vs Absorption.
From spiff.rit.edu
Emission and Absorption Lines Light Emission Vs Absorption The result is an emission spectrum that shows the intensity of emission as a function of wavelength. infographic showing the relationship between the continuous spectrum of a star whose light is shining on gas, the emission spectrum of. during absorption, an electron takes energy from an incoming photon, and the internal energy of the electron increases. Absorption and. Light Emission Vs Absorption.